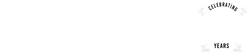Recently on Capitol Hill in Washington, DC, Ron DiGiaimo, CEO of RCCS (together with the Association of Cancer Care Centers) led a targeted advocacy effort focused on transforming cancer care.
The delegation met with legislators to discuss pressing issues in the oncology field. Aiming to convey the critical needs and opportunities for legislative action to improve patient care and treatment outcome.
|
Categories
All
Archives
July 2024
|
|
DO YOU HAVE A QUESTION?
WE HAVE AN ANSWER. Office: 512.583.2000
Fax: 512.583.2002 |
FOLLOW THE LATEST INDUSTRY TRENDS
|
CELEBRATING 25 YEARS
|
@2024 Revenue Cycle Coding Strategies. All rights reserved.